Diabetic Complications
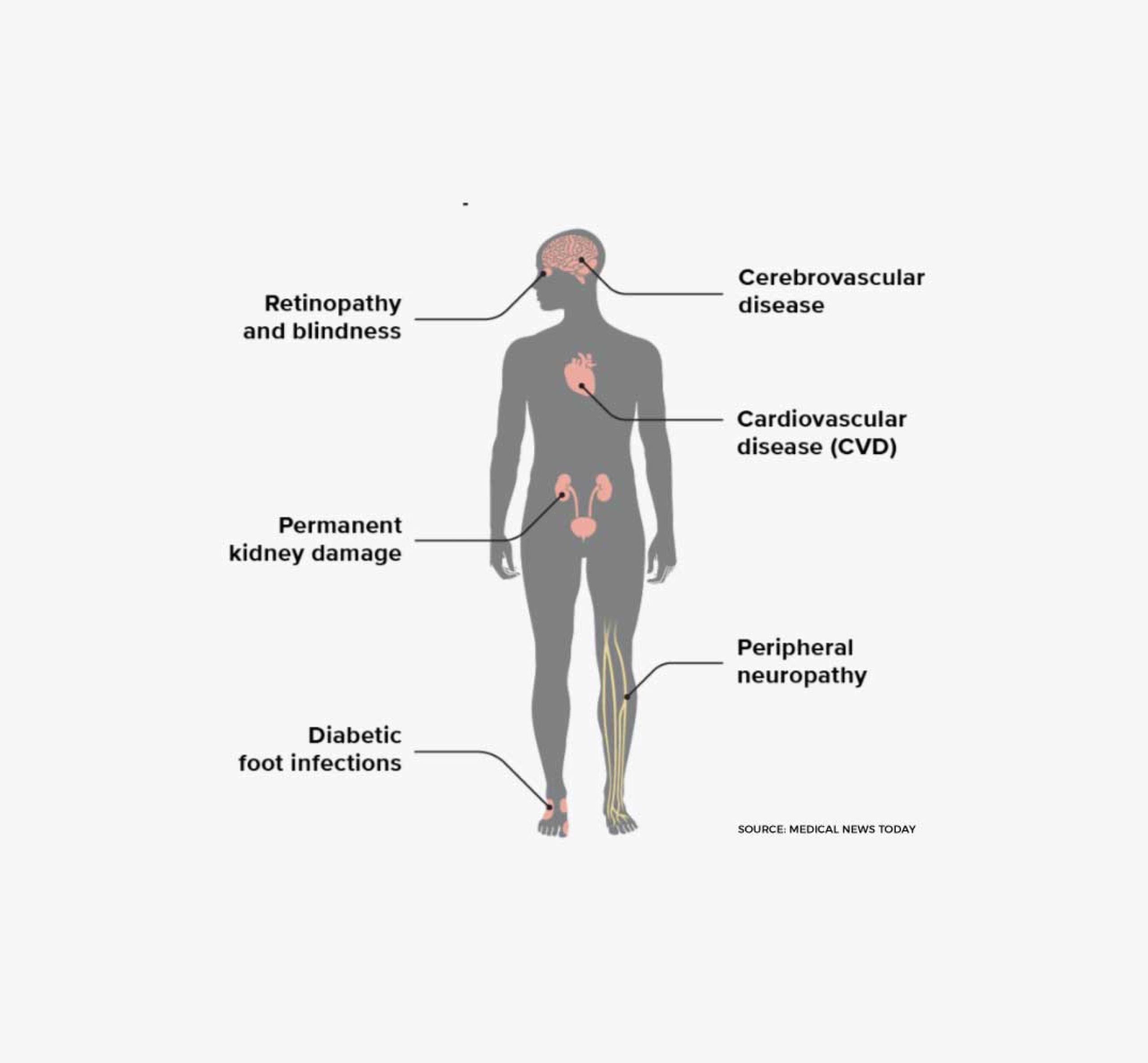
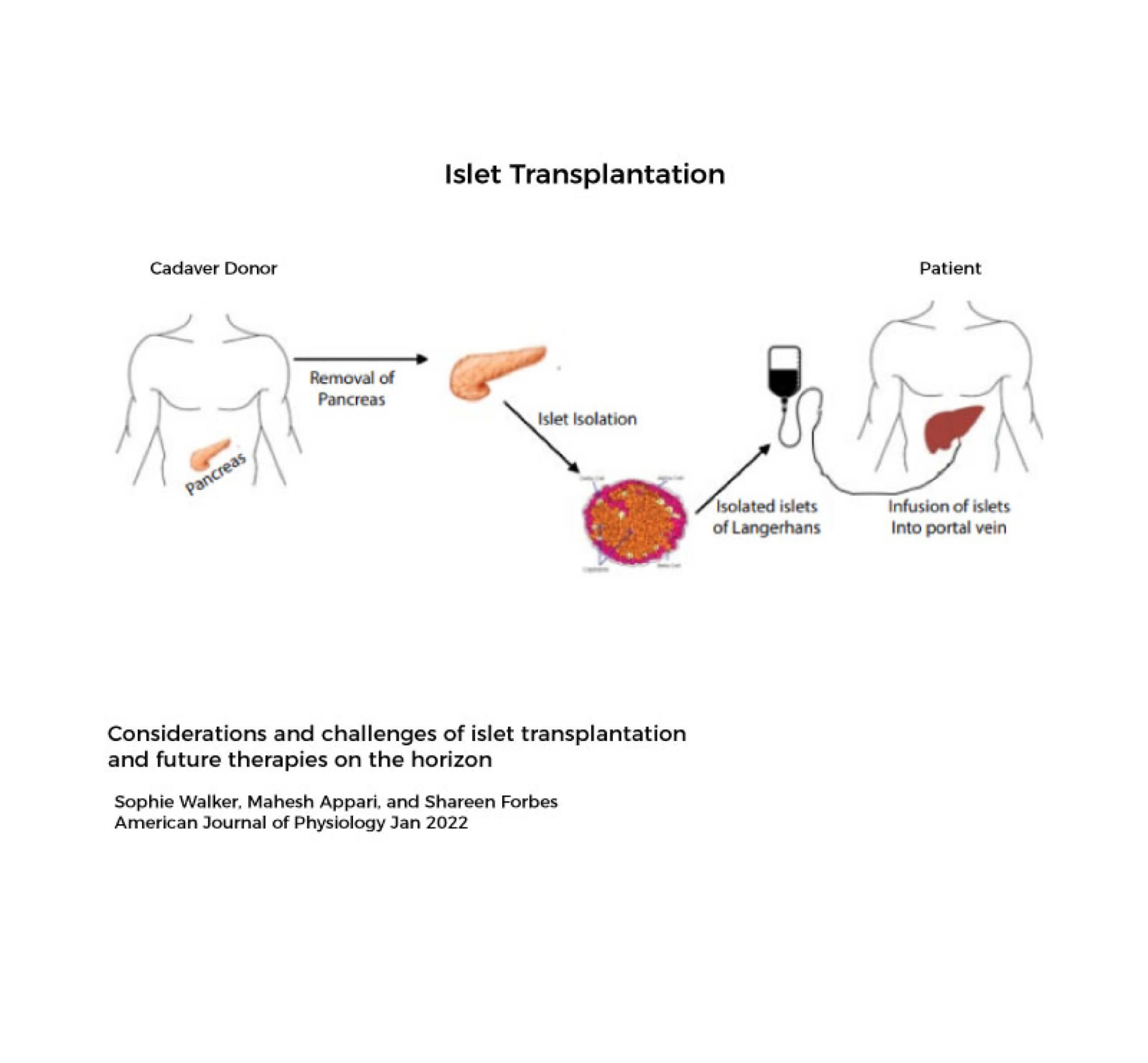
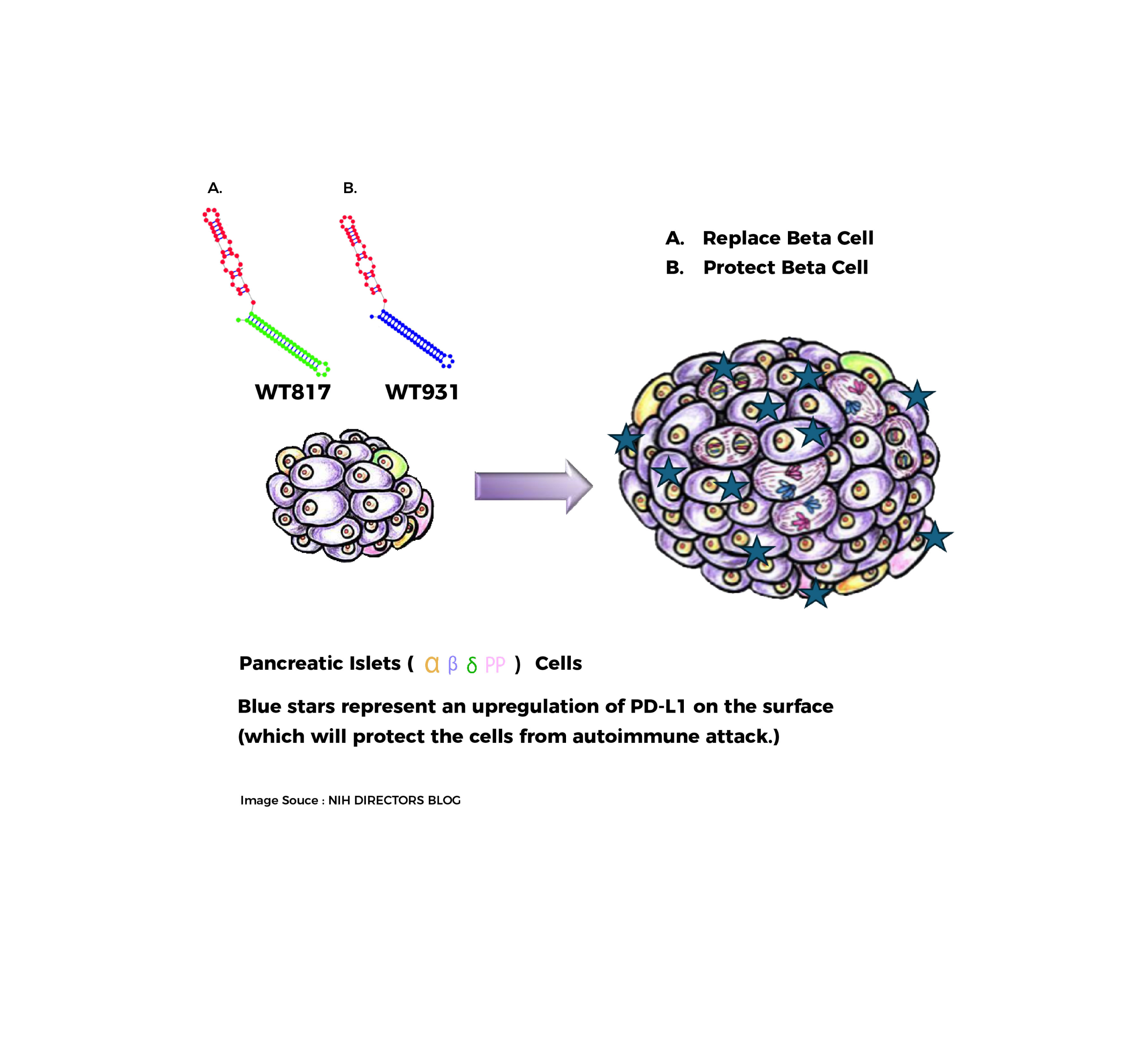
There currently is no cure for Type 1 Diabetes. The standard of care for patients with Type 1 Diabetes is to manage the disease through lifelong insulin replacement therapy, typically administered via multiple daily injections or an insulin pump, combined with careful monitoring of blood sugar levels through diet, exercise, and regular glucose testing. Type 1 diabetes can lead to many long term health complications.
Average lifespan shortened by approximately 12 years, similar to the average decrease caused by all cancers.
Source: Mortality in Type 1 Diabetes in the Current Era Michelle Katz, MD, MPH1,2,3; Lori Laffel, MD, MPH1,3 JAMA Jan 2015